The Mass Percent Calculator simplifies complex calculations, making it easier for students, educators, and professionals to analyze chemical compositions accurately and efficiently.
In the world of chemistry, understanding the composition of compounds is crucial. This is where the Mass Percent Calculator becomes an indispensable tool. It helps in determining the mass percent composition of each element in a compound – a fundamental concept in chemistry that has significant applications in both academic and industrial settings.
What is Mass Percent Calculator?
Mass Percent Calculator is a useful tool that estimates the mass percentage of compounds within substances. This formula calculates their mass percentage as a proportion to that of all ingredients present. Mass percents can also be used in chemical analysis to ascertain concentrations of substances within solutions.
The formula for calculating mass percent is:
Mass Percent (M%) = (Mass of Compound/Mass of Total Substance) * 100.
M% stands for mass percent of a compound, MC for its total mass and MS for its substance mass.
To explain the formula, mass percent is calculated by dividing the mass of a compound by its total mass and then multiplying that result by 100 to get an answer expressed as a percentage. For instance, if a sample contains 4 grams of compound in 20 grams of mixture, its mass percent would be:
M% = (4g / 20g)*100 = 20%
This means the compound accounts for 20% of the overall mass of a mixture.
Mass Percent Definition:
Mass percent, also known as percent by mass or weight percent, is a measurement of the mass of a solute in a given amount of solution, expressed as a percentage. It is calculated by dividing the mass of the solute by the mass of the solution and then multiplying by 100. Mass percent is commonly used in chemistry to express the concentration of a solution or the composition of a mixture.
Understanding the Mass Percent Formula
The formula for calculating mass percent is relatively straightforward:
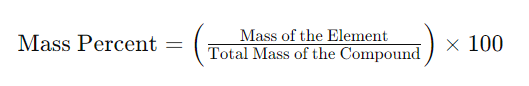
- Mass of the Element: This is the mass of the individual element within the compound.
- Total Mass of the Compound: This is the sum of the masses of all the elements in the compound.
How to Calculate Mass Percent?
To calculate the mass percent of a compound in a substance, follow these steps:
- Calculate the mass of a compound in grams.
- Subtract its total mass from that amount for total mass.
- Divide this result by 100 to get its percentage value expressed as mass as grams.
- Consider a solution containing 10 grams of salt dissolved in 90 grams of water.
For example, suppose a solution contains 10 grams of salt dissolved in 90 grams of water.
To calculate the mass percent of salt in the solution:
- The mass of salt is 10 grams.
- The total mass of the solution is 90 grams plus 10 grams, or 100 grams total mass.
- Dividing 10 grams by 100 gives us 0.1
- Multiplying that figure by 100 gives 10%, so our solution contains 10 grams of salt by its overall mass.
Definition and Background of Mass Percent
Mass percent is a term commonly used in chemistry to express the concentration of an element in a compound or a component in a mixture. It is defined as the mass of a specific component divided by the total mass of the mixture or compound, multiplied by 100%. This concept is essential in stoichiometry for determining the composition of substances and in analytical chemistry for preparing solutions with precise concentrations.
Step-by-Step Calculation Guide
- Determine the Masses: Find the mass of the element and the total mass of the compound. For example, in a water molecule (H₂O), if the mass of Hydrogen is 2 grams, and the total mass of the molecule is 18 grams.
- Input the Values: Enter 2 in the ‘Mass of Element’ field and 18 in the ‘Total Mass of Compound’ field.
- Calculate and Interpret: Click ‘Calculate’. The calculator will display 11.11%, meaning hydrogen constitutes 11.11% of the water molecule by mass.
Table of Example Calculations
| Compound | Element | Mass of Element | Total Mass of Compound | Mass Percent |
|---|---|---|---|---|
| H₂O | H | 2 g | 18 g | 11.11% |
| CO₂ | C | 12 g | 44 g | 27.27% |
| NaCl | Na | 23 g | 58.5 g | 39.32% |
Glossary for Mass Percent
- Mass Percent: The concentration of a component expressed as a percentage of the total mass.
- Compound: A substance formed from two or more elements chemically bonded together.
- Element: A substance that cannot be broken down into simpler substances by chemical means.
- Stoichiometry: The part of chemistry that deals with the relative quantities of reactants and products in chemical reactions.
FAQ Section
Q: Why is mass percent important in chemistry? A: Mass percent helps in understanding the composition of compounds and is crucial for tasks like preparing solutions and analyzing chemical reactions.
Q: Can mass percent be greater than 100%? A: No, mass percent values are always between 0% and 100%, as they represent a part of the whole.
Q: How is mass percent different from molarity? A: Mass percent is a measure of concentration based on mass, while molarity is based on the number of moles of a solute per liter of solution.
This comprehensive guide on Mass Percent Calculator provides a blend of practical utility and theoretical knowledge, making it a valuable resource for anyone dealing with chemical compositions.