The Atoms to Grams Calculator is an invaluable tool for students, educators, and professionals in these fields. This calculator simplifies the complex process of converting the total number of atoms of a substance to grams, and vice versa. It is useful for calculating molecular weights for chemical reactions and determining quantities for laboratory experiments.
Understanding the relationship between the number of atoms and their mass in grams is a fundamental concept in chemistry and physics. Atoms to Grams calculator is designed to provide accurate, quick, and easy conversions.
How to Use the Atoms to Grams Calculator
- Input the Number of Atoms: If you wish to convert atoms to grams, enter the total number of atoms of the substance.
- Input the Average Atomic Mass: Enter the average atomic mass of the substance in atomic mass units (amu).
- Choose the Conversion: Click on “Convert Atoms to Grams” for converting atoms to grams, or “Convert Grams to Atoms” for the reverse conversion.
- View the Result: The result will be displayed in the result section. For atoms to grams conversion, the result will be in grams, and for grams to atoms, the result will be the number of atoms.
- Reset for New Calculation: Use the reset button to clear all inputs and results for a new calculation.
Explaining the Formula Used in the Atoms to Grams Calculator Converter
The Atoms to Grams Calculator relies on Avogadro’s number and the concept of the mole in chemistry. Avogadro’s number, approximately 6.022×10236.022×1023, represents the number of atoms or molecules in one mole of a substance.
The formulae used in the calculator are:
Converting Atoms to Grams:
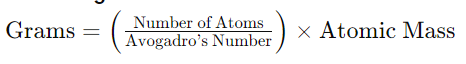
This formula calculates the mass in grams of a given number of atoms, based on the atomic mass of the substance.
Converting Grams to Atoms:
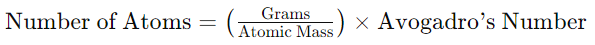
This formula calculates the number of atoms in a given mass of a substance, using the atomic mass and Avogadro’s number.
Definition and Background of Atoms and Grams
Atoms are the smallest units of matter that retain the identity of a chemical element. Each atom has a defined atomic mass, which is a measure of its weight. Grams, a unit of mass in the metric system, are used to quantify the amount of substance. The relationship between atoms and grams is central to chemical stoichiometry, which is the study of the quantitative relationships between the reactants and products in a chemical reaction.
Step-by-Step Calculation Guide for Atoms to Grams
Calculating Atoms to Grams:
Example: Convert 2 moles of Carbon atoms (atomic mass = 12 amu) to grams.
- Step 1: Determine the number of atoms.
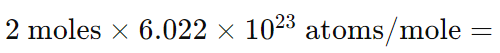

- Step 2: Apply the formula
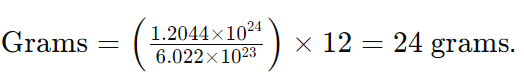
Table of Example Calculations for Atoms to Grams
| Substance | Atomic/Molecular Mass (amu) | Quantity (Atoms/Molecules) | Quantity (Grams) |
|---|---|---|---|
| Carbon | 12 | 6.022×10236.022×1023 (1 mole) | 12 grams |
| Oxygen | 16 | 3.011×10233.011×1023 (0.5 moles) | 8 grams |
| Water | 18 | 6.022×10236.022×1023 (1 mole) | 18 grams |
Glossary for Atoms to Grams Calculator
- Atom: The smallest unit of a chemical element.
- Gram: A unit of mass in the metric system.
- Atomic Mass Unit (amu): A unit used to express atomic and molecular weights.
- Avogadro’s Number: The number of constituent particles (usually atoms or molecules) in one mole of a substance, approximately 6.022×10236.022×1023.
- Mole: A unit in chemistry representing Avogadro’s number of atoms or molecules.
Real-World Applications of Atom to Gram Calculator Conversions across Industries
Atom to Gram Calculator in Pharmaceutical Industry
In the pharmaceutical sector, the Atoms to Grams Calculator plays an integral role in drug formulation and development. Precise atom-to-gram conversions are essential for ensuring accurate dosages of active pharmaceutical ingredients (APIs).
This precision directly impacts the efficacy and safety of medications. For instance, in the synthesis of complex molecules used in drugs, each step requires the exact measurement of reactants and products. The calculator aids chemists in determining the precise amount of a substance required at the molecular level, thereby facilitating the creation of effective and safe pharmaceutical products.
Material Science
Material science is another area where the Atoms to Grams Calculator finds significant application. In this field, understanding the atomic composition of materials is vital for developing new materials with desired properties.
For example, in the creation of alloys or composite materials, the exact proportion of each element can drastically affect the material’s characteristics such as strength, flexibility, and conductivity. The calculator assists material scientists in quantifying these proportions at the atomic level, enabling them to innovate and improve materials for various applications.
Environmental Analysis
The environmental sector also benefits from the precise conversions provided by the Atoms to Grams Calculator. In environmental analysis, accurately determining the atomic or molecular concentration of pollutants in air, water, or soil is crucial for assessing environmental health and compliance with safety standards.
The calculator enables scientists to convert measurements taken in grams to atoms or molecules, providing a clearer understanding of the concentration levels of various contaminants, which is essential for effective environmental monitoring and remediation efforts.
Nanotechnology
Finally, in the burgeoning field of nanotechnology, the calculator is indispensable for manipulating matter at the nanoscale. Nanotechnology often involves working with atoms and molecules directly, and the ability to convert between the mass of substances in grams and the number of atoms is critical.
This precision is essential for designing and synthesizing nanomaterials and nanostructures with specific properties, which have applications ranging from electronics to medicine. The Atoms to Grams Calculator thus becomes a key tool in the nanotechnologist’s toolkit, enabling groundbreaking advancements in this cutting-edge field.
In summary, the Atoms to Grams Calculator is not just a theoretical tool but a practical asset across various industries, playing a pivotal role in advancing scientific and technological innovations. Its ability to provide accurate conversions between the mass and the atomic scale is fundamental in driving precision, safety, and innovation in these crucial sectors.
Historical Perspective on Atomic Mass Measurement
The journey of understanding and measuring atomic mass is a fascinating tale that stretches from the mystical practices of alchemy to the precise calculations of modern quantum chemistry. This evolution reflects humanity’s growing understanding of the fundamental building blocks of matter and our increasing capability to measure them with accuracy.
The Alchemy Era
The story begins in the era of alchemy, a precursor to modern chemistry, flourishing from the Middle Ages to the Renaissance. Alchemists, driven by a blend of mysticism, philosophy, and early scientific endeavors, sought to transform base metals into noble ones like gold.
While their understanding of atoms and elements was primitive, they laid the groundwork for the concept of distinct substances with unique properties – a stepping stone towards the idea of atomic mass.
Dalton’s Atomic Theory
A significant leap occurred with John Dalton in the early 19th century. Dalton, often regarded as the father of modern atomic theory, proposed that each element is composed of unique atoms and that the mass of these atoms could differentiate them.
His work introduced the concept of relative atomic masses, providing the first systematic method to assess the masses of atoms compared to a standard, which he arbitrarily set as hydrogen, the lightest element.
The Periodic Table and Standardization
The mid-19th century saw Dmitri Mendeleev’s creation of the Periodic Table, arranging elements by increasing atomic mass and similar chemical properties. This arrangement highlighted the periodicity of elements and further stressed the importance of atomic mass in understanding chemical behavior.
The need for standardization led to the adoption of oxygen as the reference standard for atomic mass, replacing hydrogen due to its greater stability and abundance.
The Advent of Modern Techniques
The 20th century ushered in groundbreaking advancements. The discovery of isotopes, atoms of the same element with different masses, by Frederick Soddy, added complexity to atomic mass measurement.
The mass spectrometer, developed in the 1910s, allowed for the precise determination of atomic and isotopic masses. This period also saw the shift from oxygen to carbon-12 as the standard reference for atomic mass, facilitating greater consistency and accuracy in measurements.
Quantum Chemistry and Beyond
Today, in the realm of quantum chemistry, atomic mass measurement is not just about weighing atoms but understanding their behavior at a subatomic level. Advanced techniques like high-resolution mass spectrometry and computational methods have enabled scientists to measure atomic masses with extraordinary precision. These measurements are critical in various scientific fields, from drug development to the study of fundamental particles.
In conclusion, the measurement of atomic mass has evolved from rudimentary guesses in the era of alchemy to precise calculations in modern quantum chemistry. This journey mirrors humanity’s relentless pursuit of understanding the fundamental nature of matter, showcasing our progress from mystical practices to scientific rigor.
FAQ Section
Q: What is Avogadro’s number and why is it important in this calculator? A: Avogadro’s number is the number of atoms or molecules in one mole of a substance. It’s crucial for converting between atoms and grams, providing a link between the atomic scale and the macroscopic scale of matter.
Q: Can I use this calculator for molecules as well as atoms? A: Yes, the calculator can be used for molecules by using the molecular mass instead of the atomic mass.
Q: How accurate are the conversions in this calculator? A: The conversions are highly accurate, assuming the atomic or molecular mass is accurately known and entered.
Additional Resources for Atoms to Grams and Grams to Atoms Calculators and Conversions
We found several useful online resources that can help you understand and perform atom to gram and gram to atom conversions:
- Savvy Calculator’s Atoms To Grams Calculator: This tool provides an easy way to convert the number of atoms of an element to its mass in grams. It uses the formula where mass in grams is calculated by multiplying the number of atoms with atomic weight and dividing by Avogadro’s number. The site also offers examples and a FAQ section for further clarification (Savvy Calculator).
- BYJU’S Grams to Atoms Calculator: This online calculator facilitates the conversion from grams to atoms. It requires the atomic mass or molar mass and the amount in grams to perform the conversion. The website explains the process with an example and provides a simple interface for calculations (BYJU’S).
- Calculator-Online.net’s Grams To Atoms Calculator: This calculator allows for both grams to atoms and atoms to grams conversions. It also explains the concept of Avogadro’s number and the formulas used for these conversions. It includes examples to demonstrate how the conversions are done (Calculator-Online.net).
- Ezcalc.me’s Grams to Atoms Calculator: This resource offers a calculator for converting grams to atoms and vice versa. It provides a user-friendly interface where you can input the necessary values to get quick conversions (Ezcalc.me).
- Sciencing’s Guide on How to Convert Atoms to Grams: This article explains the concepts involved in converting atoms to grams, including Avogadro’s number and molar mass. It provides a step-by-step guide on performing these calculations manually, which can be useful for understanding the underlying principles (Sciencing).
Each of these resources offers unique features and explanations that can be helpful for different aspects of understanding and performing these conversions.

