The pOH Calculator simplifies the process of determining the pOH of a solution, a fundamental aspect of assessing its acidity or basicity. This guide is designed to provide a detailed overview of the pOH calculator, its functionality, and its significance in various applications.
Calculating the pOH (power of hydroxide ion concentration) is essential in chemistry, especially when dealing with solutions and their basicity. The pOH Calculator simplifies this process by allowing you to quickly determine the pOH value based on the hydroxide ion concentration. Understanding the pOH of a solution is crucial in the field of chemistry, especially when dealing with acid-base reactions.
The relationship between pH and pOH is crucial for grasping the basics of acid-base chemistry, and this calculator makes these calculations straightforward and accessible. By inputting either the pH or pOH value, users can quickly obtain the corresponding pOH or pH, streamlining studies and practical applications in chemistry.
Learn more about this Calculator
- 1 How to Use the pOH Calculator: A Detailed User Guide
- 2 Formula used for pOH Calculator
- 3 Understanding pOH: Definition and Background
- 4 Table and a Chart for various pOH Conversion Examples
- 5 Glossary of Key Terms Related to pOH and pOH Calculator
- 6 pOH Calculator FAQs
- 7 Additional Online Resources about pOH and pOH Calculator
How to Use the pOH Calculator: A Detailed User Guide
The pOH Calculator is a simple, user-friendly tool designed to help you calculate the pOH value from the pH value of a solution and vice versa. This guide will walk you through each step of using the calculator, ensuring you can efficiently determine the pOH or pH values for your needs.
First, ensure you have access to a device with an internet connection and a web browser. The pOH Calculator is web-based, so no additional software installation is necessary.
Open your web browser and navigate to the pOH Calculator. The calculator should load on your screen, presenting a clean interface with input fields for pH and pOH values, a reset button, and an area where your calculation results will be displayed.
pOH Calculator User Interface Overview
The calculator interface comprises the following elements:
- pH Value Input: A numeric input field where you can enter the pH value of your solution.
- pOH Value Input: A numeric input field for entering the pOH value, should you need to calculate the corresponding pH instead.
- Reset Button: Clears all input fields and results, allowing you to start a new calculation.
- Result Display: Shows the calculated pOH or pH value after you enter the corresponding value in one of the input fields.
How to Calculate pOH from pH
- Enter the pH Value:
- Locate the input field labeled “pH Value.”
- Type in the pH value of your solution. Ensure that the value is between 0 and 14, as this is the valid range for pH.
- Automatic Calculation:
- Once you enter the pH value, the calculator automatically computes the corresponding pOH value.
- The result will be displayed under the input fields, labeled as “pOH.”
How to Calculate pH from pOH
- Enter the pOH Value:
- Find the input field labeled “pOH Value.”
- Input the pOH value of your solution, making sure it falls within the 0 to 14 range.
- Automatic Calculation:
- After you’ve entered the pOH value, the calculator will automatically determine the pH value for you.
- Look for the result displayed as “pH” under the input fields.
Resetting the pOH Calculator
If you wish to perform another calculation or clear the current values:
- Click the Reset Button.
- This action clears both the pH and pOH input fields and any results displayed, resetting the calculator for a new calculation.
- The focus will automatically move to the pH input field, ready for you to enter a new value.
Tips for Accurate Results
- Ensure that the values entered are accurate and within the 0-14 range. Values outside this range are considered invalid and will not produce a result.
- Use the reset button to clear previous calculations before starting a new one to avoid confusion.
pOH Calculator Troubleshooting
- Invalid Input: If you enter a value outside the 0-14 range, the calculator will prompt you to enter a valid value. Check your input and try again.
- Browser Issues: Ensure your web browser is up to date. Older versions may not support the calculator’s functionality.
The pOH Calculator offers a straightforward solution for quickly converting between pH and pOH values. Whether you’re a student, educator, or professional in the field of chemistry, this tool simplifies the calculation process, allowing you to focus on your work without manual computations.
Formula used for pOH Calculator
To understand the relationship between pH and pOH, and how they are calculated, let’s break down the formulas and concepts involved in more detail. The calculations are based on the properties of aqueous solutions at 25°C (298 K), where the ion product of water (Kw) is
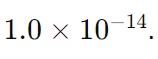
The formulas relating pH and pOH are as follows:
Formula to calculate pOH from pH:
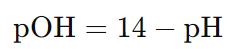
Formula to calculate pH from pOH:
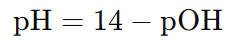
These formulas are derived from the relationship:

This is because at 25°C,

and taking the negative logarithm of both sides gives:
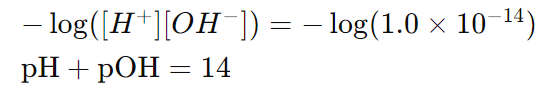
Here,

is the concentration of hydrogen ions in moles per liter, and
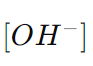
is the concentration of hydroxide ions in moles per liter. pH is the negative logarithm (base 10) of the hydrogen ion concentration, and pOH is the negative logarithm (base 10) of the hydroxide ion concentration.
This relationship is a fundamental concept in acid-base chemistry and is used to calculate the acidity or basicity of a solution. The pOH Calculator simplifies these calculations by instantly converting pH values to pOH values and vice versa, based on the inputs provided.
To understand the relationship between pH and pOH, and how they are calculated, let’s break down the formulas and concepts involved in more detail.
Understanding pOH: Definition and Background
The concept of pOH serves as a metric for understanding the basicity of an aqueous solution, standing as a complement to the more commonly referenced pH scale, which measures acidity. Originating from the field of chemistry, pOH quantifies the level of basicity through a logarithmic scale, similar in structure to the pH scale but focused on the presence of hydroxide ions.
The scale ranges from 0 to 14, where a lower value indicates a solution with higher basicity, and vice versa. This method of measurement is integral to various scientific disciplines, including biochemistry and environmental science, as it provides crucial insights into the chemical properties of substances.
The development of the pOH scale has enabled scientists to achieve a more nuanced understanding of aqueous solutions, facilitating the precise manipulation of their acidic or basic conditions for a wide array of applications, from industrial processes to the intricate balances within biological systems. The equilibrium between pOH and its acidic counterpart highlights the interconnectedness of acidic and basic properties, emphasizing the dynamic nature of chemical solutions.
The Importance of pOH in Chemistry
Understanding pOH is critical in the field of chemistry, where the balance between acidic and basic components can influence the outcome of chemical reactions. The ability to measure and adjust the basicity of a solution allows chemists to control reaction environments, ensuring that processes proceed under optimal conditions.
This control is particularly important in reactions that are sensitive to the concentration of hydroxide ions, as even slight variations in basicity can significantly impact the rate and direction of a reaction. Additionally, the concept of pOH plays a crucial role in the study of buffer solutions, which are designed to maintain a stable pH level despite the addition of acids or bases.
Through the application of pOH, chemists can formulate solutions that resist changes in pH, providing a stable environment for biochemical processes and industrial applications alike.
pOH in Environmental Sciences
In environmental sciences, the measurement of pOH offers valuable insights into the health and composition of natural water bodies. By assessing the basicity of lakes, rivers, and oceans, scientists can identify changes in water chemistry that may indicate pollution, eutrophication, or other ecological disturbances. For instance, a shift towards higher basicity could suggest the presence of alkaline industrial waste, while lower basicity levels might be indicative of acid rain impact.
Through continuous monitoring of pOH levels, environmental scientists can track the effects of human activities on aquatic ecosystems, enabling the development of strategies to mitigate pollution and restore water quality. This application underscores the broader significance of pOH beyond the laboratory, highlighting its role in addressing some of the most pressing environmental challenges of our time.
pOH and Biological Systems
The regulation of pOH is also vital in biological systems, where the balance between acidity and basicity can affect physiological processes and the functionality of cellular components. Many enzymes, for example, require specific pH levels to function optimally, and by extension, the pOH levels can influence these conditions.
The body’s ability to maintain a stable internal pH, a process known as homeostasis, is partly dependent on mechanisms that manage the concentration of hydroxide ions. Disruptions in this balance can lead to conditions such as alkalosis or acidosis, which can have significant health implications.
Therefore, the study of pOH not only contributes to our understanding of chemical reactions but also plays a crucial role in the fields of medicine and biology, where it helps to elucidate the intricate balance required for life.
Table and a Chart for various pOH Conversion Examples
To illustrate the concept of pOH and its relevance across different contexts, the following table provides a variety of examples, showcasing how pOH values can vary in different solutions and under various conditions. This table aims to give a broad perspective on the practical applications and implications of pOH in chemistry, environmental sciences, and biology.
| Example Context | Solution/Condition | pOH Value Range | Implications/Relevance |
|---|---|---|---|
| Laboratory Acids and Bases | 0.1 M NaOH (Sodium Hydroxide) | Around 1 | Indicates high basicity; commonly used in titrations and pH adjustments in chemical experiments. |
| 0.1 M HCl (Hydrochloric Acid) | Around 13 | Reflects very low basicity; used to study the behavior of acids and bases. | |
| Natural Water Bodies | Pure Water at 25°C | 7 | Neutral basicity; serves as a reference point for comparing other solutions. |
| Ocean Water | 7.5 – 8.4 | Slightly basic; indicates the presence of bicarbonates and carbonates balancing marine pH. | |
| Environmental Monitoring | Acid Rain-affected Lake | 8 – 9 | Higher basicity may indicate buffering capacity or pollution effects. |
| Industrial Wastewater | 10 – 12 | Very high basicity; suggests contamination with alkaline substances. | |
| Biological Systems | Human Blood | 7.4 (pH) | Slightly basic, essential for maintaining physiological functions. |
| Cytoplasm of Human Cells | Around 7.2 (pH) | Slightly basic, optimal for cellular processes and enzyme activity. |
This table captures the diversity of pOH values across different scenarios, emphasizing its utility in assessing the basicity of solutions in various scientific fields. From the controlled conditions of a chemistry lab to the complex dynamics of natural and biological systems, understanding pOH values helps in interpreting chemical behaviors, environmental health, and biological processes.
It’s crucial to note that while pOH provides insights into basicity, it is often considered in conjunction with pH values for a comprehensive understanding of a solution’s acidity-basicity balance.
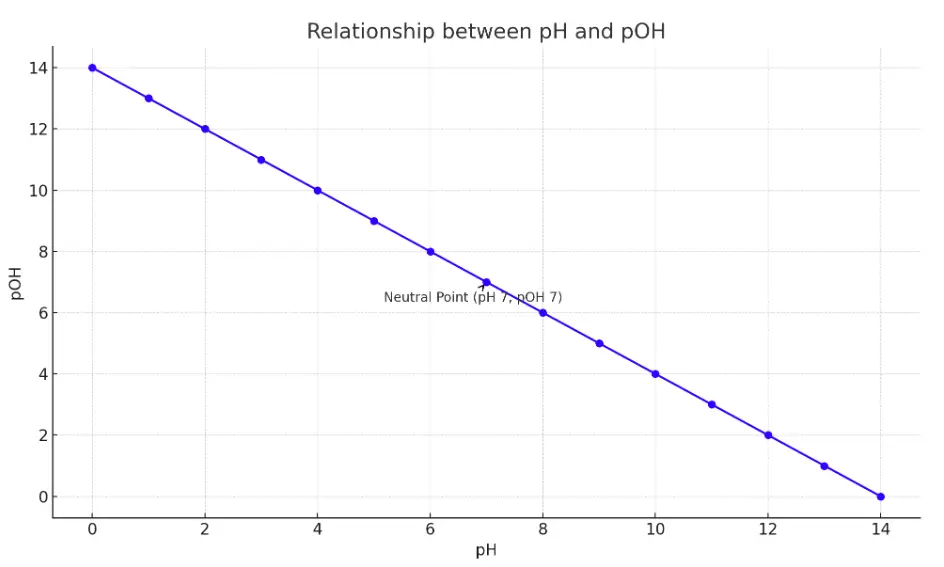
The chart above illustrates the inverse relationship between pH and pOH across the pH scale from 0 to 14. As the pH increases, indicating a decrease in acidity, the pOH decreases, reflecting an increase in basicity. This inverse correlation is highlighted by the linear relationship between pH and pOH values.
The chart marks a neutral point at pH 7 and pOH 7, where the solution is neither acidic nor basic, exemplifying the balance of hydrogen and hydroxide ions in pure water at 25°C. This graphical representation serves as a visual aid to understand how changes in pH reflect opposite changes in pOH, underlying the fundamental concepts of acid-base chemistry.
Glossary of Key Terms Related to pOH and pOH Calculator
Acid: A substance that increases the concentration of hydrogen ions ((H^+)) in a solution, typically having a pH less than 7.
Alkalosis: A condition characterized by an overly basic pH in the body’s fluids, often due to an excess of bicarbonate or a loss of hydrogen ions.
Base (or Alkali): A substance that increases the concentration of hydroxide ions ((OH^-)) in a solution, typically having a pH greater than 7.
Buffer: A solution that resists changes in pH when acids or bases are added to it, typically consisting of a weak acid and its conjugate base.
Hydrogen Ion ((H^+)): A positively charged ion or proton, the concentration of which determines the acidity of a solution.
Hydroxide Ion ((OH^-)): A negatively charged ion, the concentration of which determines the basicity of a solution.
Ion Product Constant for Water ((K_w)): The product of the concentrations of hydrogen ions and hydroxide ions in water, which is (1.0 \times 10^{-14}) at 25°C.
Neutral: A condition where the concentration of hydrogen ions equals the concentration of hydroxide ions in a solution, typically indicated by a pH of 7.
pH: A logarithmic scale used to specify the acidity or hydrogen ion concentration of an aqueous solution, ranging from 0 to 14.
pOH: A logarithmic scale used to specify the basicity or hydroxide ion concentration of an aqueous solution, also ranging from 0 to 14.
Titration: A laboratory method used to determine the concentration of an acid or base in a solution by adding a titrant of known concentration until the reaction reaches its endpoint, often indicated by a pH change.
Acid Rain: Precipitation with a pH lower than normal, resulting from atmospheric pollution that causes environmental harm, typically affecting water bodies and leading to a change in their pOH levels.
Eutrophication: A process driven by the enrichment of water bodies with nutrients (nitrogen and phosphorus), leading to excessive growth of algae and other aquatic plants, affecting the pH and pOH balance of the ecosystem.
Homeostasis: The self-regulating process by which biological systems maintain stability while adjusting to conditions that are optimal for survival.
These terms form the foundation of understanding the concepts related to pOH, acidity, and basicity in aqueous solutions, highlighting the interconnectedness of chemical principles that govern both natural and artificial environments.
pOH Calculator FAQs
Q1: What is pOH, and why is it important?
A1: pOH is a measure of the concentration of hydroxide ions in a solution. It’s important because it helps determine the basicity or alkalinity of a solution, complementing the pH scale, which measures acidity.
Q2: Can I use this calculator for solutions other than aqueous solutions?
A2: Yes, you can use it for any solution where you know the hydroxide ion concentration in moles per liter.
Q3: What is the relationship between pOH and pH?
A3: The pH and pOH of a solution are related by the equation pH + pOH = 14 at 25°C. So, if you know one, you can easily calculate the other.
Q4: What happens if I enter a negative hydroxide ion concentration?
A4: The calculator won’t work with negative concentrations, as it’s not physically meaningful.
Q5: Can I calculate pOH for concentrated solutions as well?
A5: Yes, you can calculate pOH for solutions with both low and high hydroxide ion concentrations.
Additional FAQ about pOH and pOH Calculator Topics
FAQ: Understanding pOH and Using a pOH Calculator
1. What is pOH?
pOH is a measure of the basicity of a solution. It quantifies the concentration of hydroxide ions ((OH^-)) in a solution, providing an inverse measure of acidity similar to the pH scale but focused on basicity.
2. How is pOH calculated?
pOH is calculated using the formula: pOH = -log(([OH^-])), where ([OH^-]) is the concentration of hydroxide ions in moles per liter (M). In a pOH calculator, you input the hydroxide ion concentration to get the pOH value.
3. What is the relationship between pH and pOH?
The relationship between pH and pOH is described by the equation: pH + pOH = 14. This equation holds true for aqueous solutions at 25°C, indicating a balance between acidity and basicity in water.
4. How does temperature affect pOH?
Temperature affects the ion product constant of water ((K_w)), which in turn influences both pH and pOH values. As temperature increases, (K_w) also increases, leading to lower pOH (and pH) values for neutral water.
5. Can pOH be greater than 14 or less than 0?
In theory, pOH values should range between 0 and 14 for aqueous solutions at 25°C. However, extreme conditions can lead to pOH values slightly outside this range, especially at high temperatures or with strong concentrations of hydroxide ions.
6. What is the significance of a pOH value of 7?
A pOH value of 7 indicates a neutral solution at 25°C, where the concentration of hydroxide ions is equal to the concentration of hydrogen ions, each being (1.0 \times 10^{-7}) M.
7. How do I use a pOH calculator?
To use a pOH calculator, enter the concentration of hydroxide ions in your solution. The calculator will apply the pOH formula to compute the pOH value, providing an instant measure of the solution’s basicity.
8. What applications does pOH have in real life?
pOH is important in various fields, including environmental science, where it helps assess water quality; chemistry, for reaction optimization; and biology, for understanding physiological processes influenced by basicity.
9. Why is it important to measure both pH and pOH?
Measuring both pH and pOH provides a complete picture of a solution’s acid-base balance. This dual measurement is crucial for applications requiring precise control over a solution’s chemical properties, such as in pharmaceuticals and biotechnology.
10. How does pOH relate to acid-base titrations?
In acid-base titrations, pOH changes as a base is added to an acid (or vice versa), helping to identify the equivalence point. Monitoring pOH alongside pH allows for precise determination of the endpoint of the titration.
11. What is a buffer, and how does it relate to pOH?
A buffer is a solution that resists changes in pH when small amounts of acid or base are added. It relates to pOH by maintaining a stable hydroxide ion concentration, thus ensuring a stable basicity level against external changes.
12. Can I determine the concentration of hydroxide ions from pOH?
Yes, you can determine the concentration of hydroxide ions by rearranging the pOH formula: ([OH^-] = 10^{-pOH}). This calculation is often performed to understand the basicity of a solution in chemical terms.
These FAQs aim to clarify the concept of pOH and its practical applications, providing a foundational understanding for students, educators, and professionals dealing with chemical solutions.
The pOH Calculator simplifies the calculation of pOH, making it easier for students, chemists, and researchers to determine the basicity of solutions. It’s a valuable tool for anyone working with chemical solutions and provides quick and accurate results.
Additional Online Resources about pOH and pOH Calculator
I found a few informative online sources that delve into the topic of pOH and its calculation, offering a broad understanding of its significance in chemistry:
- Science Notes and Projects provides a comprehensive explanation of what pOH is, including its definition, calculation methods, and its relationship with pH. This source is valuable because it breaks down the concept of pOH in an easy-to-understand manner and provides examples of how to calculate it from hydroxide ion concentration or from a known pH value. Visit Science Notes and Projects.
- Khan Academy offers an extensive article on pH, pOH, and their scales, including examples and explanations of how to calculate pOH and its importance in understanding the acidity or basicity of solutions. This resource is beneficial for its educational approach to the subject, catering to both beginners and those seeking to consolidate their knowledge. Explore Khan Academy’s article.
- ThoughtCo presents a review of pOH calculations, explaining how to calculate pOH from the hydroxide ion concentration and how it relates to pH. This source stands out for its clear, concise explanations and practical examples, making complex concepts more accessible. Read ThoughtCo’s review.
Each of these sources was chosen for their clear explanations, practical examples, and authoritative insights into the topic of pOH, offering a well-rounded understanding for those interested in chemistry.

